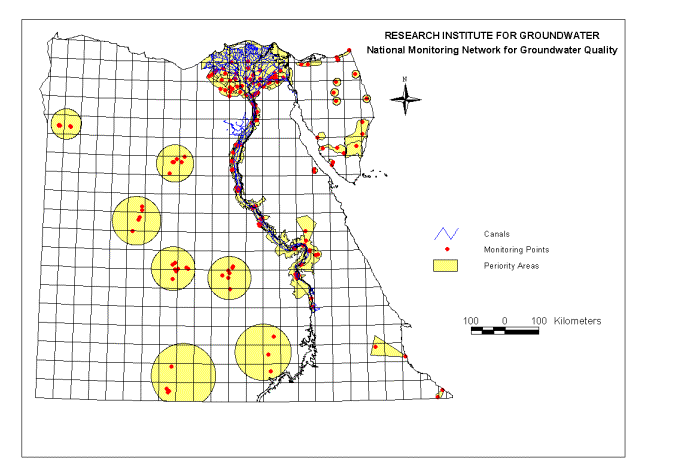
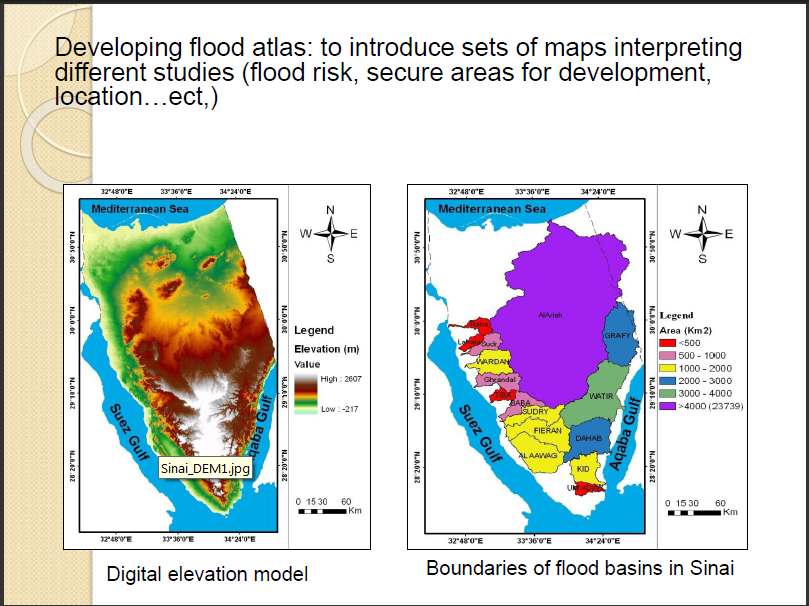
Adsorption of organochlorine pesticides on modified porous Al30/bentonite: Kinetic and thermodynamic studies
In a joint cooperation, Dr. Sleem Al-Sayed Gaber - Associate Professor) and Chem. Mohamed Sobhy Mohamed Assistant Researcher at the Central Laboratory for Environmental Quality Monitoring (CLEQM) of the National Water Research Center (NWRC) published a joint research paper entitled: Adsorption of organochlorine pesticides on modified porous Al30/bentonite: Kinetic and thermodynamic studies in the reputable journal: Arabian Journal of Chemistry. The adsorption of pesticides (heptachlor epoxide, dieldrin and endrin) onto modified bentonite by Keggin cation [Al30O8(OH)56(H2O)24]18+denoted Al30 cation to form composite (Al30/B), has been investigated as a possible alternative method for their removal from aqueous solutions. Hence, the objective of the current study is to use a low-cost material as a step towards cleaner environment. Interestingly, these chemical modifications altered the physicochemical characteristics of bentonite in term of morphology, surface area and functionality which has been confirmed by using nitrogen adsorption desorption isotherm, scanning electronic microscopy (SEM) and X-ray diffraction (XRD). Gas chromatography coupled to mass spectrometry (GCMS) was used to identify and analyze the pesticides. Different physicochemical parameters were analyzed: contact time, adsorbent dose, pH, and temperature. The results showed that the removal percentage of pesticides on Al30/B was the highest at contact time of 5?h, adsorbent dosage of 25?mg, at pH 7.5, and at optimum temperature of 45°C. Furthermore, the Kinetic study indicated that the adsorption of pesticides on Al30/B was well adapted to the pseudo-first order kinetic with a correlation coefficient near unity. The results of adsorption were fitted to the Langmuir and Freundlich isotherms. The Freundlich model represented the adsorption process better than Langmuir model, with correlation coefficients (R2) values range from 0.986 to 0.989. The Thermodynamic study suggested that the adsorption of pesticides was chemisorption, spontaneous and endothermic process. Therefore, Al30/B composite can be utilized effectively for removal of pesticides with efficiency up to 98%. Crossref DOI link: https://doi.org/10.1016/J.ARABJC.2020.06.027